WHO Suspends Sputnik V Approval Process Over Manufacturing Breaches
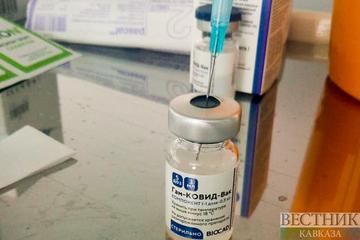
The World Health Organization (WHO) has suspended its approval process for Russia’s Sputnik V coronavirus vaccine, pending a fresh inspection of at least one Russian factory manufacturing the shot, The Moscow Times reported.
Speaking at a press briefing of the Pan American Health Organization, a regional branch of the WHO, Assistant Director Jarbas Barbosa said Russia’s bid for emergency authorization had been put on hold after a number of manufacturing infringements were uncovered during a WHO inspection in Russia in May.
“The process for Sputnik V’s emergency use listing (EUL) was suspended because while inspecting one of the plants where the vaccine is being manufactured, they found the plant was not in agreement with best manufacturing practices,” Barbosa said Wednesday.
 Latest news
Latest newsThe Use of the “Oreshnik” Missile and a New Phase of Escalation Around Ukraine
09.Jan.2026
Solidarity Deferred: Croatia and Romania’s Dangerous Retreat
08.Jan.2026
Azerbaijan’s Eurasian Initiative: Ambitions, Challenges, and Doubts
07.Jan.2026
The Great Rotation: Personnel Reshuffles in Ukraine’s Leadership
06.Jan.2026
The United States Did Not Confirm an Alleged Ukrainian Attack on Putin’s Residence
05.Jan.2026
The Trans-Caspian Fiber Optic Cable: A Digital Milestone Connecting Europe and Asia
04.Jan.2026
Georgia Hopes for a Review of Venezuela’s Recognition of Abkhazia and South Ossetia Amid Ongoing Crisis
04.Jan.2026
Ukraine’s Allies Discuss Security and the Future of a Peace Settlement
03.Jan.2026
Iran Amid a Growing Domestic Crisis: Causes, Dynamics, and External Factors
03.Jan.2026
The South Caucasus in the Context of Expanding External Involvement
02.Jan.2026

 14 Jan 2026
14 Jan 2026








